In October, the first patient was enrolled in a Phase 3 clinical trial to investigate the safety and efficacy of a polyclonal anti-SARS-CoV-2 hyperimmune globulin (CoVIg hyperimmune) drug. The hyperimmune drug will be used in the clinical trial to treat patients with severe complications resulting from COVID-19. (Read the full press release here).
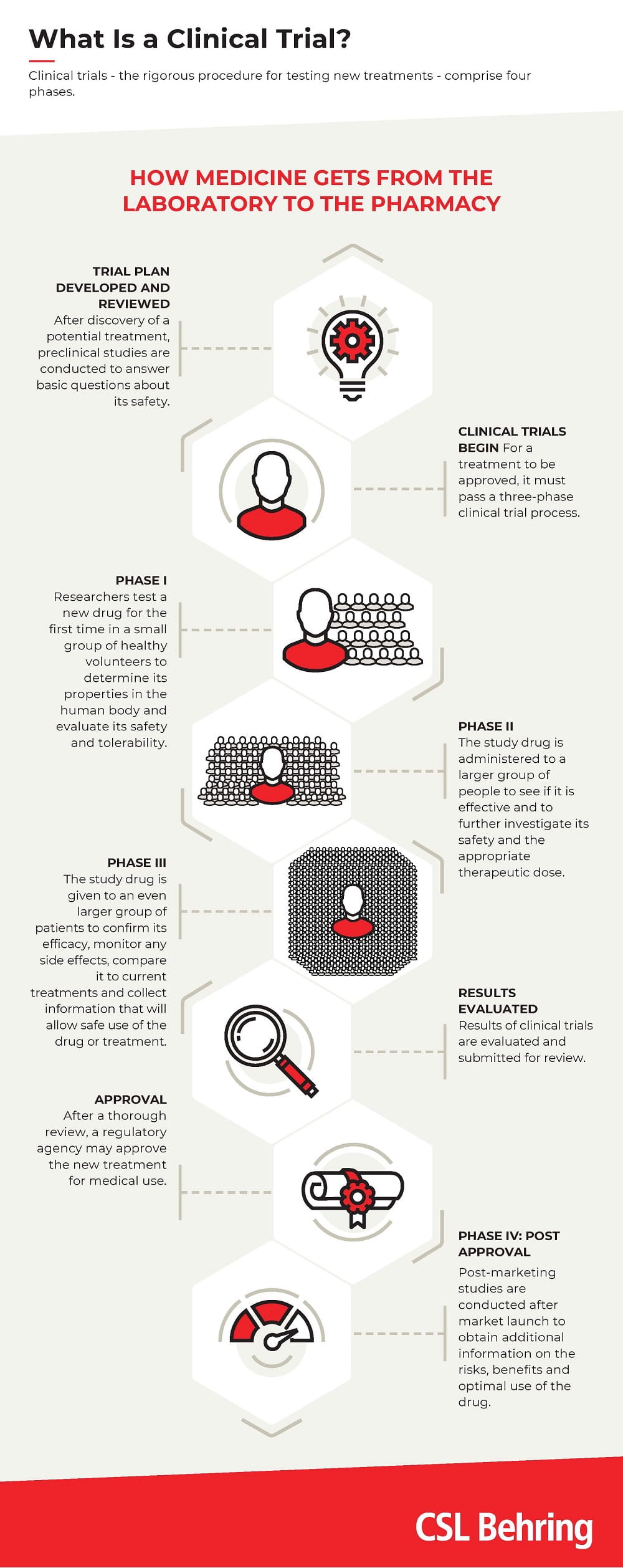
Clinical trials - the rigorous procedure for testing new treatments - consist of four phases (see diagram). Each phase of a clinical trial serves a specific purpose, but phase III is particularly important. This is because in phase III trials, the study drug is administered to a larger group of people. This depends on various criteria, such as the type of disease or the therapeutic effect. In phase III, for example, the number of patients that are needed in order to confirm the efficacy of the drug, monitor any side effects, compare it with common treatments and gather information that will enable the drug to be used safely can quickly run into several thousands.
Accelerated procedure thanks to tried-and-tested manufacturing processes
Normally, the first three phases of clinical testing of a new drug take a lot of time and it takes years to obtain marketing approval. However, in the case of the development of CoVIg Hyperimmune, it is possible to draw on production processes that have already been tested and clinical trials that have already been carried out, because the new CoVIg Hyperimmune can be manufactured in the same way as a product that is already available on the market and that is used for the treatment of immunodeficiency diseases.For this reason and thanks to the special approval of the FDA (U.S. Food & Drug Administration) and the support of the U.S. National Institutes of Health (NIH) it was possible to skip clinical phases I and II and move directly to the decisive phase III, thereby gaining valuable time in the fight against coronavirus. The NIH is a research institution that conducts clinical trials in the USA on behalf of the U.S. Department of Health and Human Services.
What is a clinical trial?
The four phases of new product development are the same worldwide(see diagram). The resulting data are used by health authorities to decide on the approval of new therapies in their respective countries.At the beginning of the year, a large group of leading plasma companies, with CSL Behring and Takeda as founding members, launched the CoVIg-19 Plasma Alliance, with the aim of advancing the development and provision of a hyperimmune drug in the global fight against COVID-19. This summer, clinical doses of the potential drug were manufactured at the CSL Behring plant in Bern and sent to the site in Marburg, Germany, for packaging.